Item no.: P4120360
Principle
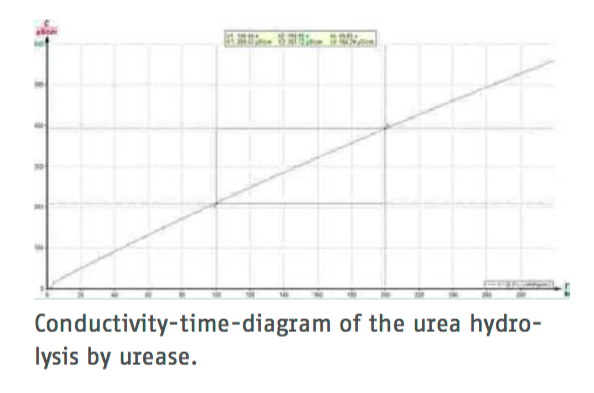
The enzymatic hydrolysis of urea in aqueous solution liberates carbon dixide and ammonia. The ions of these compounds increase the conductivity of the solution. Conductivity measurements can so be made to determine the rate of hydrolysis of urea by the enzyme urease at various substrate concentrations.
Tasks
The Michaelis constant can then be calculated from these values.
What you can learn about
- Michaelis constant
- Enzymatic hydrolysis of urea
- Conductivity measurement
- Bodenstein principle
- Enzyme-substrate complex
- Lineweaver-Burk plot
Software included. Computer not provided.