Item no.: P3020461
Principle
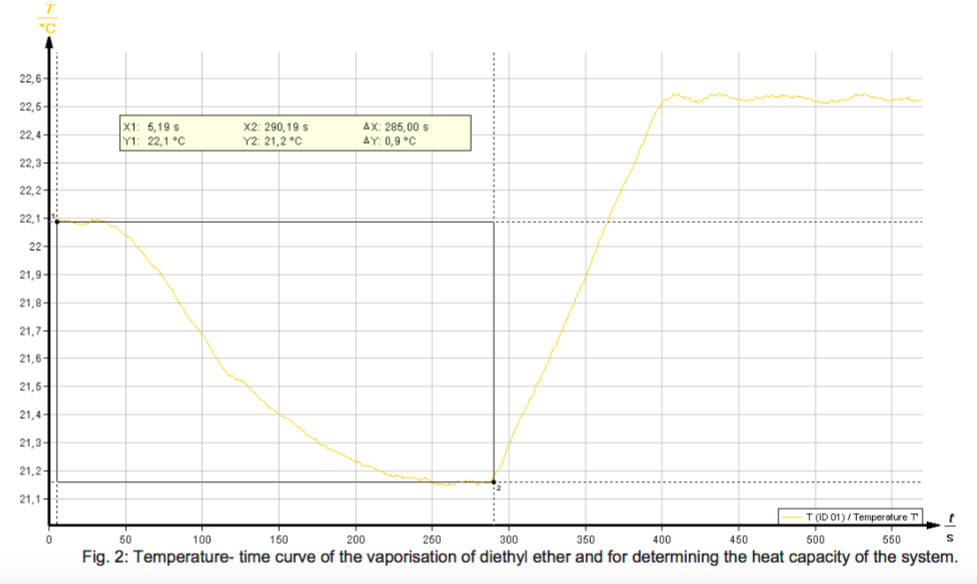
The vaporisation of a liquid occurs with heat absorption. To determine the enthalpy of vaporisation, a known mass of the liquid which is to be investigated is vaporised in a special vaporisation vessel in a current of air. The quantity of heat absorbed, which corresponds to the enthalpy of vaporisation, can be calorimetrically determined.
Tasks
- Determine the molar enthalpy of vaporisation of diethyl ether and methanol.
- Calculate the molar entropies of vaporisation and discuss the results under consideration of Trouton’s rule.
What you can learn about
- Enthalpy of vaporisation
- Enthalpy of condensation
- Enthalpy pf sublimation
- Vapour pressure
- Entropy of vaporization
- Clausius-Clapeyron equation
- Trouton’s rule
- Law of thermodynamics
- Calorimetry
Necessary accessories
- Precision balance 620g/0.001g
- Precision balance 6200g/0.01g
Software included. Computer not provided.